History
-
- Expansion of R&D CRO business and venture into new business verticals for growth.
2025 -
- LIIMS installed and NABL approval of analytical lab (in progress)
2024 -
- Upgrade of R&D lab and GLP level analytical lab expansion
- Formulation and Analytical CRO operations
- Nabota (inj.) Approval (100IU)
2021 -
- Nabota Sales started
2019 -
- eCTD System installed
2018 -
- Tech Transfer 1 Case
- EGF approval submission for India
- Nabota (inj) (50IU)
2017 -
- CA-DMF Submission for UDCA (Canada)
- Tech Transfer 1 Case
- Nabota (inj.) Approval (100IU)
2016 -
- US-DMF submission
- Tech Transfer 1 case
- Expansion
- Meropenem approval (Korea’s 1st)
- Whole Sale License
2015 -
- Tech Transfer 3 cases
- MeropenemA NDA Submission
- Establishment of Indian Corporation
2012 -
- R&D Operation Started (first Korean Company)
- Tech Transfer 2 cases
2009 -
- Elevation to Subsidary
2007 -
- Contact office (Liaisoning)
2006
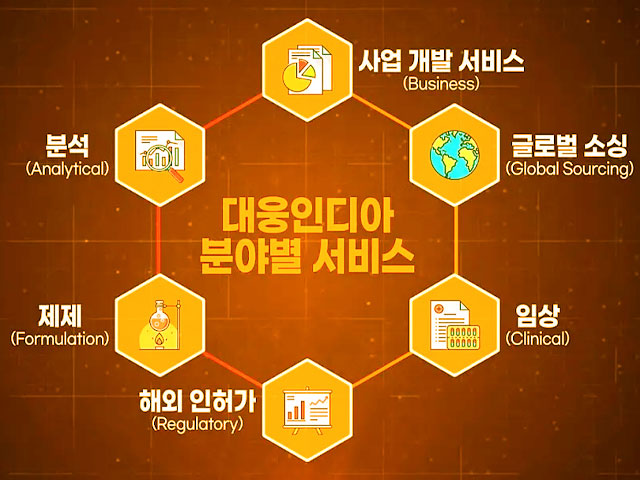
Who we are
Total Solution Provider
- Formulation
- Analytical
- Clinical
- Regulatory
- Global Sourcing
- Business

Vision
To provide total solutions to all the pharma needs and contribute to improving quality of life of patients.

Mission
To provide the most beneficial total solutions with pharmaceuticals and services that contribute to improving quality of life of valued consumers.
Core Values
Justice: Never take an unrighteous path even if it can bring profits.
Fairness: Think in the shoes of others and never lean too much to one side.
Win-Win: Only do work that is the interest of us, counterparts and the society (Win-Win-Win).
Open Mind: Always speak the truth and listen to other opinions with an open mind.
Stewardship: Unite work and fate and work hard until being successful.

Accreditations
ISO
NABL
Wholesale license 20B
Wholesale license 21B



Daewoong way
Direction that Daewoong should take and the culture in which Daewoong employees work.

Growth
Our goal is to help you 'grow' as the industry's top human resource.

Autonomy
We provide an environment where you can 'autonomously' immerse yourself in work.

Performance
We focus on working properly to exhibit 'performance'.
Director's Message
Daewoong Pharmaceutical ventured into India with the vision of leveraging the skills and talents of the Indian pharmaceutical market to support the globalization of the company, not only across Asia but also in regulated markets such as America and Europe. Our objective has always been to connect globally by harnessing the expertise and opportunities offered by the Indian pharmaceutical industry, thereby establishing ourselves as a leading global pharmaceutical company.
The journey began in 2006 with the establishment of a liaison office, which was elevated to a subsidiary status in 2007. Subsequently, in November 2012, Daewoong Pharmaceutical (India) Pvt. Ltd. was established in Hyderabad. This entity was created to position itself as a Formulation and Analytical Services Contract Research Organization, catering to both global and Indian domestic markets. Over time, we have developed a research center in India and expanded our business to include services tailored to our customers’ needs, such as research, development, production, and authorization—all built upon the core competencies we have accumulated over the years.Daewoong India has further diversified its business verticals, including clinical services support in collaboration with Indian CROs, regulatory affairs, sourcing of bulk and fine chemicals from India, custom synthesis, and manufacturing. Additionally, we provide business development support in India and its neighboring regions to multinational corporations (MNCs).
In line with the Indian government’s strong support for the biopharmaceutical market, we have introduced Nabota and plan to launch products such as epidermal growth factors and growth hormones in the near future. Recognizing the increasing interest in healthcare driven by India’s economic growth, we aim to expand our product offerings and enhance the Daewoong brand through strategic business partnerships in the medical instruments and cosmetics sectors.
We sincerely thank all our customers for their continued interest in our services. We remain committed to delivering the highest quality of service, backed by the knowledge and expertise we have gained over the past 18 years. We look forward to serving you with even greater dedication and excellence in the years to come.
As we embark on a new beginning in 2025, I extend my heartfelt wishes for a happy and prosperous New Year to all!
